
The validity of the approximation is discussed and it is concluded that the time-dependence dR 2/ dt = − kD is reasonable for the case of θ-phase precipitates in Al + 4 wt.-% Cu alloy. The dissolution of a spherical precipitate, taking account of transient effects,is solved using an approximation for the diffusion field. The result of Thomas and Whelan is for three-dimensional diffusion and is obtained essentially from the steady-state part of the diffusion field around a spherical precipitate. These equations can be used to represent what happens in precipitation reactions. schemes is determined by the interplay between the terms in Equation 5. This form exists, to show which reactants and products are just floating in the solution, and which of them actually react. Aaron's result is essentially one-dimensional and is derived from the transient part of the diffusion field in one dimension. The first step to writing a net ionic equation is to separate the soluble (aqueous) reactants and products into their respective cations and anions. In precipitate cutting regimes during creep, planar faults such as APBs and. 2AgNO 3 (aq) + Na 2 S (aq) -> Ag 2 S (s) + 2NaNO 3 (aq) But, that's not the end of precipitate equations - there's still one last step: turning it into a net ionic equation. It is pointed out that the “disagreement” arises because the situations treated are themselves dissimilar. Guidelines for Writing Net Ionic Equations Step I.
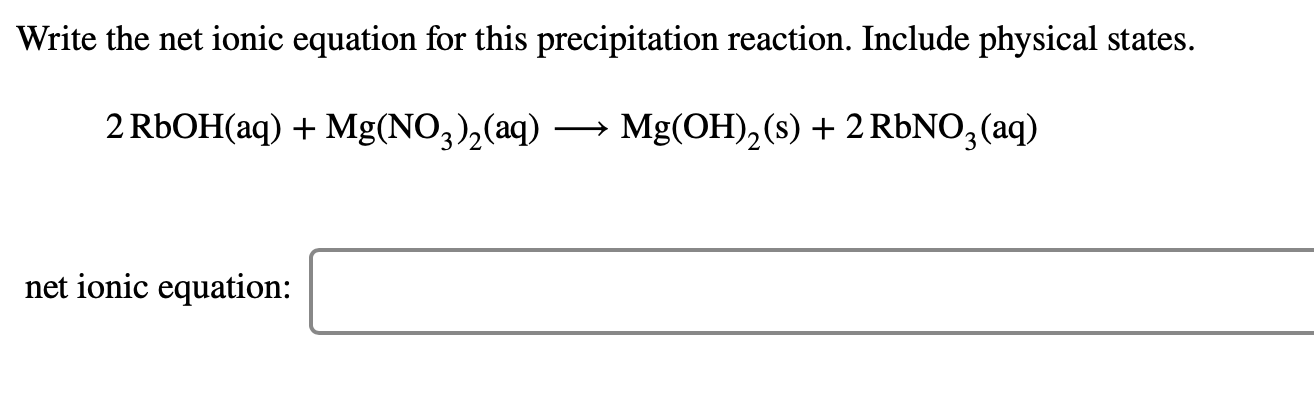
If there is a precipitation reaction, write the complete and net ionic equation that describes the reaction.

Moreover, the time-dependence of the radius of a dissolving precipitate according to Aaron (R=R0−KDt) disagrees with that of Thomas and Whelan ( dR 2/ dt = − kD). equation remains the net ionic equation that includes only the substances and ions that actually remains in the reaction as water, gas, insoluble solid (precipitate), weak electrolyte, and no electrolyte. EXAMPLE 1 Predicting Precipitation Reactions: Predict whether a precipitate will form when water solutions of silver nitrate, AgNO 3 (aq), and sodium sulfide, Na 2 S(aq), are mixed. Mag., 1961, 6, 1103), where dissolution was considered to be approximately the reverse of growth, is in error in this assumption. J., 1968, 2, 192) in which it is implied that a previous treatment by Thomas and Whelan ( Phil.
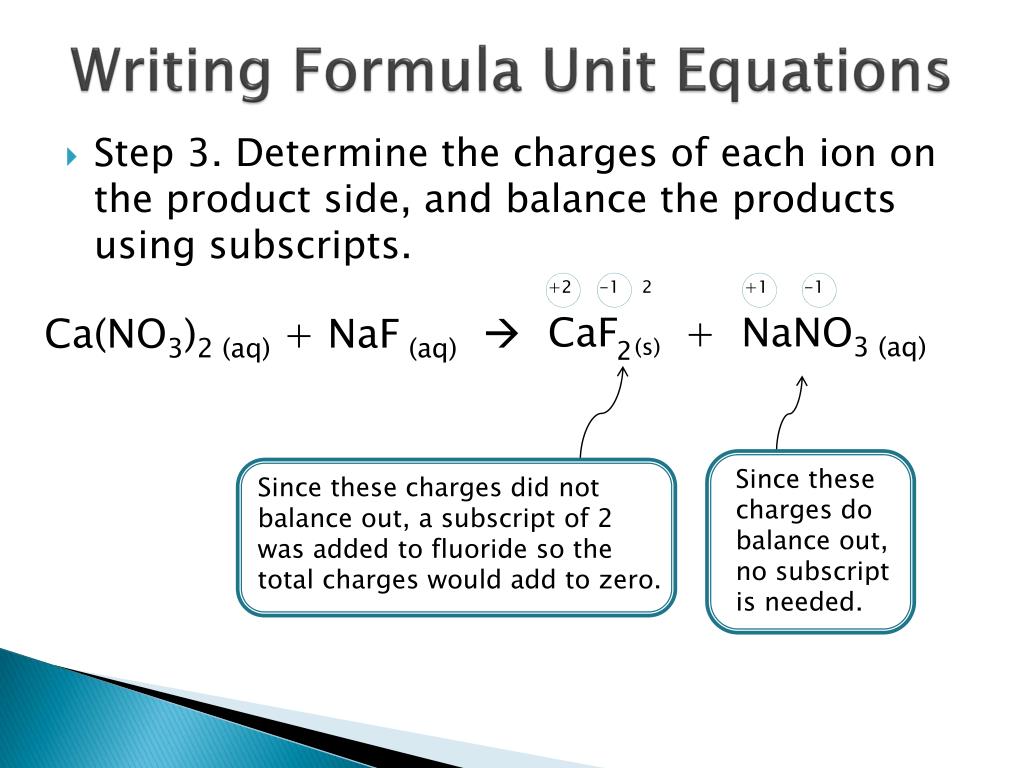
A theory of precipitate dissolution has recently been proposed by Aaron ( Metal Sci.


 0 kommentar(er)
0 kommentar(er)
